By: Eunice Jean Patron
Thermoresponsive polymers are molecules that undergo significant changes in their properties in response to temperature changes and are widely used in biomedicine, such as drug delivery, tissue growth, and gene transfer, as noted by researchers Ward and Georgiou in their 2011 study.
One example of a thermoresponsive polymer is poly(N-isopropylacrylamide), which is used to deliver drugs, such as calcitonin and insulin, to their target organ. According to scientist Dirk Schmaljohann, poly(N-isopropylacrylamide) keeps the drug intact as it passes through the stomach, and once it reaches the intestines, which have a different pH level, the polymer breaks down and releases the drug.
While there are already existing conventional methods to synthesize polymers, it is difficult to control how monomers—small molecules that make up polymers—connect. This leads to varying polymer chain lengths, which affect the performance of the polymers. This prompted chemists from the University of the Philippines – Diliman College of Science (UPD-CS) to identify a simple, inexpensive, and environmentally friendly approach of creating thermoresponsive polymers.
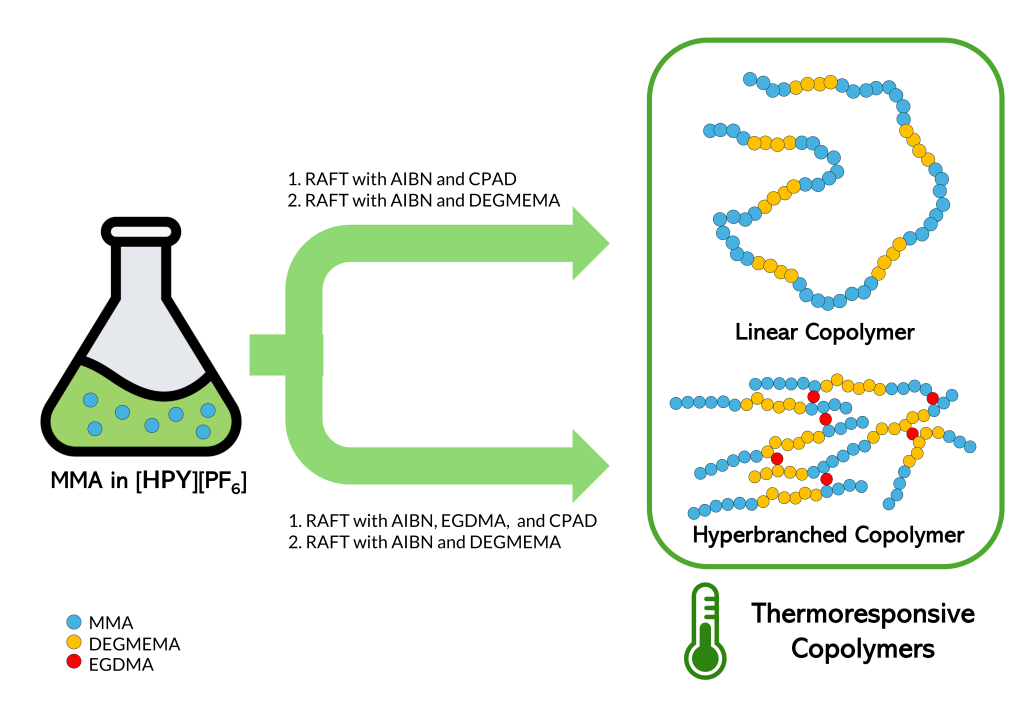
“RAFT polymerization helps control the growth of polymer chains by mitigating the formation of chains that can no longer grow (a.k.a. ‘dead’ polymers), thereby creating a narrower molecular weight distribution which can allow more tailored polymer designs or properties,” Madrid explained. Solvents used in RAFT polymerization are also typically organic and volatile, posing environmental and health risks. To lessen these risks, the researchers used [HPY][PF6], a type of ionic liquid that has better polymerization kinetics and is less harmful to the environment.
Apart from demonstrating the effectiveness of synthesizing polymers with [HPY][PF6] through RAFT polymerization, the study introduced new possibilities in polymer research. “The results open up the potential for using other hexylpyridinium ionic liquids to create dual-responsive polymers (e.g. responsive to both temperature and pH), which are valuable for biomedical applications such as drug delivery,” Madrid concluded.
For interview requests and other concerns, please contact [email protected].
References:
Madrid, L. L., Perez, S. J., & Arco, S. (2024). RAFT copolymerization of methyl methacrylate and di(ethylene glycol) methyl Ether methacrylate in a hexylpyridinium Ionic liquid. Journal of the Chinese Chemical Society. https://doi.org/10.1002/jccs.202400197
Schmaljohann, D. (2006). Thermo- and pH-responsive polymers in drug delivery. Advanced Drug Delivery Reviews, 58(15), 1655-1670. https://doi.org/10.1016/j.addr.2006.09.020
Ward, M. A., & Georgiou, T. K. (2011). Thermoresponsive polymers for biomedical applications. Polymers, 3(3), 1215-1242. https://doi.org/10.3390/polym3031215
